HOUSTON – Children’s Memorial Hermann Hospital and Cord Blood Registry® (CBR) are launching the first FDA-approved, Phase I safety study on the use of cord blood stem cells to treat children with sensorineural hearing loss.
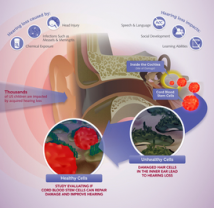 The study, which will use patients’ stem cells from their own stored umbilical cord blood, is the first-of-its-kind, and has the potential to restore hearing. This follows evidence from published laboratory studies that cord blood helps repair damaged organs in the inner ear.
The study, which will use patients’ stem cells from their own stored umbilical cord blood, is the first-of-its-kind, and has the potential to restore hearing. This follows evidence from published laboratory studies that cord blood helps repair damaged organs in the inner ear.
The year-long study will follow 10 children, ages 6 weeks to 18 months, who have sustained post-birth hearing loss.
The Principal Investigator is Samer Fakhri, M.D., surgeon at Memorial Hermann-Texas Medical Center and associate professor and program director in the Department of Otorhinolaryngology – Head & Neck Surgery at UTHealth. Linda Baumgartner, MS, CCC-SLP, Auditory-Verbal Therapist, is a co-investigator.
“Currently, the only treatment options for sensorineural hearing loss are hearing aids or cochlear implants,” Dr. Fakhri said. “We hope this study will open avenues to additional treatment options for hearing loss in children.”
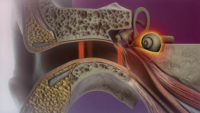
Researchers will obtain and process the patients’ stored cord blood for treatment. The cells then will be given to the patients via IV infusion and patients will be observed for several hours in the hospital.
Patients will return to the hospital to repeat all tests except the MRI at one month and one year and all tests with an MRI at six months.
“This study is exciting because it might offer a non-surgical option for some children with profound loss,” Linda Baumgartner said. “More importantly, this is the first treatment with the potential to restore normal hearing.”
Since more infants are surviving premature birth, physicians and researchers are seeing a rising number of very young children with significant hearing loss. About 15 percent of children in the U.S. also suffer from low-frequency or high-frequency hearing loss that can impact the child’s speech, language and social development and can increase their risk of developing learning disabilities, according to Dr. Fakhri.
 “We share Dr. Fakhri’s and Dr. Baumgartner’s passion and commitment to understanding more about the potential applications of cord blood to help repair nerve tissue,” said Heather Brown, vice president of scientific and medical affairs at CBR. “It is exciting to be at the forefront of research to match children who have cord blood stored, with this team of groundbreaking doctors studying autologous stem cell therapies for hearing loss.”
“We share Dr. Fakhri’s and Dr. Baumgartner’s passion and commitment to understanding more about the potential applications of cord blood to help repair nerve tissue,” said Heather Brown, vice president of scientific and medical affairs at CBR. “It is exciting to be at the forefront of research to match children who have cord blood stored, with this team of groundbreaking doctors studying autologous stem cell therapies for hearing loss.”
The study is supported by CBR and TIRR Foundation.
For information on participation in the study, visit www.cordblood.com/hearingloss.
Source: Children’s Memorial Hermann Hospital